Abstract
Due to its relict nature, the unique Baroninae swallowtail, Baronia brevicornis, is considered a “living fossil”. It is also one of the most enigmatic butterfly species with contentious origins and peculiar ecological characteristics. The aim of this study is to evaluate the genetic diversity and population structure of this endemic species of butterfly in Mexico. We sampled populations in two areas within its restricted geographical range in central Mexico and the isolated subspecies population in the state of Chiapas. Three ISSR primers produced 66 loci, indicating a high genetic diversity (P = 100 %, H e = 0.22) and variation range in these populations (62 % < P < 85 %, 0.18 < H e < 0.25). The Chiapas population presented the lowest values. The observed high values can be explained by the population dynamic of this species characterized by a very high density of individuals over very limited areas. Variation between populations appears to reflect both the age of colonization and locality perturbation level. Two methods of genetic structure analysis (Self-Organizing Map and Structure analysis) match to define three clusters. Natural and anthropogenic barriers may explain the separation between two clusters (cluster 1 and 2) of central Mexico but an unexpected result revealed that the Chiapas population is not genetically distinguishable from the central Mexico populations (cluster 3) leading us to hypothesize a possible “recent” separation or anthropogenic introduction. Habitat and host plant specificity probably limits the exchange of individuals between populations thus increasing fragmentation and leading to a complex genetic structure. We should put in place population monitoring schemes at different spatial scales, combining field occurrences and genetic tools, in order to reduce extinction susceptibility and keep track of recolonization events for this enigmatic species.



Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Aubert J, Legal L, Descimon H, Michel F (1999) Molecular phylogeny of swallowtail butterflies of the tribe papilionini (Papilionidae, Lepidoptera). Mol Phylogenet Evol 12(2):156–167
Barilani M, Sfougaris A, Giannakopoulos A, Mucci N, Tabarroni C, Randi E (2007) Detecting introgressive hybridisation in rock partridge populations (Alectoris graeca) in Greece through Bayesian admixture analyses of multilocus genotypes. Conserv Genet 8:343–354
Becerra JX (2005) Timing the origin and expansion of the Mexican tropical dry forest. PNAS 102:10919–10923
Behura SK (2006) Molecular marker systems in insects: current trends and future avenues. Mol Ecol 15:3087–3113
Caterino MS, Reed RD, Kuo MM, Sperling FAH (2001) A partitioned likelihood analysis of swallowtail butterfly phylogeny (Lepidoptera: Papilionidae). Syst Biol 50:106–127
de Jong R, Vane-Wright RI, Ackery PR (1996) The higher classification of butterflies (Lepidoptera): problems and prospects. Entomol Scand 27:65–101
De la Maza J, White J, White A (1987) Observaciones sobre el polimorfismo femenino de Baronia brevicornis Salv. (Papilionidae: Baroniinae) con la descripción de una nueva subespecies del Estado de Chiapas, México. Rev Soc Mex Lep 11:3–13
Dorado O, Arias DM, Ramírez R, Sousa M (2005) Leguminosas de la Sierra de Huautla. Centro de Educación Ambiental e Investigación Sierra de Huautla, Conabio 176 pp
Dunphy BK, Hamrick JL (2007) Estimation of gene flow into fragmented populations of Bursera simaruba (Burseraceae) in the dry-forest life zone of Puerto Rico. Am J Bot 94:1786–1794
Eisner T (2003) Living fossils: on lampreys, Baronia, and the search for medicinals. Bioscience 53:265–269
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software Structure: a simulation study. Mol Ecol 14:2611–2620
Excoffier L (2004) Patterns of DNA sequence diversity and genetic structure after a range expansion: lessons from the infinite-island model. Mol Ecol 13:853–864
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction sites. Genetics 131:479–491
Falush D, Stephens M, Pritchard JK (2007) Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol Ecol Notes 7:574–578
Haeuser CL (1992) A new abdominal scent organ in females of Baronia brevicornis (Lepidoptera: Papilionidae). Zool Anz 229:54–62
Hancock DL (1983) Classification of the Papilionidae: a phylogenetic approach. Smithersia 2:1–48
Heikkilä M, Kaila L, Mutanen M, Peña C, Wahlberg N (2012) Cretaceous origin and repeated tertiary diversification of the redefined butterflies. Proc R Soc B 279:1093–1099
Hoffman CC (1922) Restos de una Antigua fauna del Norte entre los Lepidopteros Mexicanos. Rev Mex Biol 3(1):1–23
Hundsdoerfer A, Wink M (2005) New source of genetic polymorphisms in Lepidoptera. Z Naturforsch 60:618–624
IUCN (2013) IUCN red list of threatened species. Version 2013.2. www.iucnredlist.org. Accessed 07 May 2014
Kar PK, Srivastava AK, Lokesh G, Sinha MK (2013) Population genetic analysis of raily ecorace of Antheraea mylitta Drury using RAPD markers. The Ecoscan Special Issue III:107–110
Khurad AM, Kanginakudru S, Qureshi SO, Rathod MK et al (2006) A new Bombyx mori larval ovarian cell line highly susceptible to nucleopolyhedrovirus. J Invertebr Pathol 92:59–65
Kohonen T (2001) Self-organizing maps, 3rd edn. Springer, Berlin
Leberger R (2010) Etude du polymorphisme génétique de Baronia brevicornis endémique stricte du Mexique. Master dissertation, Université Paul Sabatier, Toulouse
Legal L, Bermúdez-Torres K, Leyva Sanchez E, Machkour-M’Rabet S (2008) ISSR, una nueva herramienta para el estudio de especies y poblaciones en el marco de la genética de la conservación. Mesoamericana 12(3):74
Legal L, Dorado O, Machkour-M’Rabet S, Leberger R, Albre J, Mariano N, Gers C (2014) Ecological constraints and distribution of the primitive and enigmatic Mexican butterfly Baronia brevicornis. Can Entomol (in press)
León-Cortés JL, Pérez-Espinoza F, Marín L, Molina-Martínez A (2004) Complex habitat requirements and conservation needs of the only extant Baroniinae swallowtail butterfly. Anim Conserv 7:241–250
Luis-Martinez A, Llorente-Bousquets J, Vargas-Fernandez I, Warren AD (2003) Biodiversity and biogeography of Mexican butterflies (Lepidoptera: Papilionoidea and Hesperioidea). Proc Entomol Soc Wash 105:209–224
Luque C, Legal L, Staudter H, Gers C, Wink M (2002) ISSR (inter simple sequence repeats) as genetic markers in Noctuids (Lepidoptera, Heterocera, Noctuidae, Noctuinae). Hereditas 136:251–253
Luque C, Legal L, Machkour-M’Rabet S, Winterton P, Gers C, Wink M (2009) Apparent influences of host plant distribution on the structure and the genetic variability of local populations of the Purple Clay (Diarsia brunnea). Biochem Syst Ecol 37:6–15
Lynch M, Milligan BG (1994) Analysis of population genetic structure with RAPD markers. Mol Ecol 3:91–99
MathWorks (2001) Laboratory of information and computer science, Helsinski University of Technology (Version 6.1.0.450 Release 12.1). The MathWorks, Inc. Available from http://www.cis.hut.fi/projects/somtoolbox
Metcalfe SE, O’Hara SL, Caballero M, Davies SJ (2000) Records of Late Pleistocene-Holocene climatic change in Mexico a review. Quat Sci Rev 19:699–721
Michel F, Rebourg C, Cosson E, Decimon H (2008) Molecular phylogeny of Parnassiinae butterflies (Lepidoptera: Papilionidae) based on the sequences of four mitochondrial DNA segments. Ann Soc Entomol France 44:1–36
Miller JS (1987) Phylogenetic studies in the Papilioninae (Lepidoptera: Papilionidae). Bull Am Mus Nat Hist 186:365–512
Nazari V, Zakharov EV, Sperling FAH (2007) Phylogeny, historical biogeography, and taxonomic ranking of Parnassiinae (Lepidoptera, Papilionidae) based on morphology and seven genes. Mol Phylogenet Evol 42:131–156
Nève G, Meglécz E (2000) Microsatellite frequencies in different taxa. Trends Ecol Evol 15:376–377
Peakall R, Smouse PE (2006) GenAlEx 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Pérez-Ruíz H (1967) Estudio morfológico de los estados larvarios de Baronia brevicornis Salv. y su importancia. Folia Entomol Mex 15/16:43–44
Pérez-Ruíz H (1977) Distribución y estructura poblacional de Baronia brevicornis Salv. (Lepidoptera: Papilionidae: Baroniinae) en la República Mexicana. An Inst Biol Univ Nal Autón Méx 48:151–154
Pérez-Ruíz H, Sánchez R (1986) Algunos aspectos demográficos de Baronia brevicornis Salv. (Lepidoptera: Papilionidae, Baroniinae) en dos localidades de México. An Inst Biol Univ Nal Autón Méx (Zoología) 57:191–198
Pritchard JK, Stephens M, Donnelly PJ (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959. http://www.pritch.bsd.uchicago.edu
Radjabi R, Sarafrazi A, Tarang A, Kamali K, Tirgari S (2012) Intraspecific biodiversity of iranian local races of silkworm Bombyx mori by ISSR (inter-simple sequence repeat) molecular marker. World J Zool 7:17–22
Ray N, Currat M, Excoffier L (2003) Intra-deme molecular diversity in spatially expanding populations. Mol Biol Evol 20(1):76–86
Regier JC, Mitter C, Zwick A, Bazinet AL et al (2013) A large-scale, higher-level, molecular phylogenetic study of the insect order Lepidoptera (moths and butterflies). PLoS ONE 8(3):e58568
Ribeiro DB, Batista R, Prado P, Brown KS Jr, Freitas AVL (2012) The importance of small scales to the fruit-feeding butterfly assemblages in a fragmented landscape. Biodivers Conserv 21:811–827
Roux O, Gevrey M, Arvanitakis L, Gers C, Bordat D, Legal L (2007) ISSR-PCR: tool for population identification and genetic structure of Plutella xylostella. Mol Phylogenet Evol 43(1):240–250
Seigler DS, Ebinger JE (1988) Acacia macracantha, A. pennatula, and A. cochliacantha (Fabaceae: Mimosoidae) species complexes in Mexico. Syst Bot 13:7–15
Simonsen TJ, Zakharov EV, Djernaes M, Cotton AM, Vane-Wright RI, Sperling FAH (2011) Phylogenetics and divergence times of Papilioninae (Lepidoptera) with special reference to the enigmatic genera Teinopalpus and Meandrusa. Cladistics 27:113–137
Simonsen TJ, de Jong R, Heikkilä M, Kaila L (2012) Butterfly morphology in a molecular age—does it still matter in butterfly systematics? Arthropod Struct Dev 41:307–322
Sinama M, Dubut V, Costedoat C, Gilles A et al (2011) Challenges of microsatellite development in Lepidoptera: Euphydryas aurinia (Nymphalidae) as a case study. Eur J Entomol 108:261–266
Soberón JM (2010) Niche and area of distribution modeling: a population ecology perspective. Ecography 33:159–167
Soberón JM, Townsend Peterson A (2005) Interpretation of models of fundamental ecological Niches and species’ distributional areas. Biodivers Inform 2:1–10
Srivastava PP, Vijayan K, Kar PK, Saratchandra B (2011) Diversity and marker association in tropical silkworm breeds of Bombyx mori (Lepidoptera: Bombycidae). Int J Trop Insect Sci 31:182–191
Tufto J, Lande R, Ringsby TH, Engen S, Sæther BE, Walla TR, DeVries PJ (2012) Estimating Brownian motion dispersal rate, longevity and population density from spatially explicit mark-recapture data on tropical butterflies. J Anim Ecol 81:756–769
Vandewoestijne S, Nève G, Baguette M (2002) Spatial and temporal population genetic structure of the butterfly Aglais urticae L. (Lepidoptera, Nymphalidae). Mol Ecol 8:1539–1543
Vane-Wright RI (2003) Evidence and identity in butterfly systematics. In: Boggs CL, Watt WB, Ehrlich PR (eds) Butterflies: ecology and evolution taking flight. University of Chicago Press, Chicago, pp 477–513
Vázquez L, Pérez-Ruíz H (1961) Observaciones sobre la biología de Baronia brevicornis Salv. (Lepidoptera: Papilionidae: Baroniinae). An Inst Biol Univ Nal Autón Méx 32:295–311
Vijayan K, Anuradha HJ, Nair CV, Pradeep AR et al (2006) Genetic diversity and differentiation among populations of the Indian eri silkworm, Samia cynthia ricini, revealed by ISSR markers. J Insect Sci 6:30
Wahlberg N, Braby MF, Brower AVZ, de Jong R, Lee MM, Nylin S, Pierce NE, Sperling FAH, Vila R, Warren AD, Zakharov E (2005) Synergistic effects of combining morphological and molecular data in resolving the phylogeny of butterflies and skippers. Proc R Soc B 272:1577–1586
Wink M (2006) Use of DNA markers to study birds migration. J Ornithol 147:234–244
Woodburne MO (2010) The great American biotic interchange: dispersals, tectonics, climate, sea level and holding pens. J Mammal Evol 17:245–264
Wright S (1931) Evolution in Mendelian populations. Genetics 16:97–159
Yeh FC, Yang R, Boyle TJB (1999) Population genetic analysis of co-dominant and dominant markers and quantitative traits. Belg J Bot 129:157
Acknowledgments
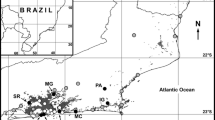
We thank Dr. Yann Hénaut from ECOSUR (el Colegio de la Frontera Sur, Chetumal, Mexico) and Celine Pélissier from the University Paul Sabatier of Toulouse (France), for their contribution in the laboratory process. Dr. Oscar Dorado (from CEAMISH, Universidad Autónoma del Estado de Morelos, Mexico) and Dr. Nestor Mariano (CIByC, Universidad Autónoma del Estado de Morelos, Mexico) are specially thanked for their valuable help in the field and useful discussions on the biology and ecology of B. brevicornis and Acacia spp. Thanks to Francisco Pérez-Espinoza for his important support in the field. Thanks to Holger Weissenberger (ECOSUR, Chetumal) for helping produce Fig. 1. We thank the “Secretaria de Medio Ambiente y Recursos Naturales” in Mexico (DOO.02-2074) for their permission to survey Baronia populations in Chiapas. Special thanks to Victor Toledo Hernández who provide us authorizations for central Mexico under the authority of the Semarnat Faut-0178 permit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Machkour-M’Rabet, S., Leberger, R., León-Cortés, J.L. et al. Population structure and genetic diversity of the only extant Baroninae swallowtail butterfly, Baronia brevicornis, revealed by ISSR markers. J Insect Conserv 18, 385–396 (2014). https://doi.org/10.1007/s10841-014-9647-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-014-9647-3
Keywords
Profiles
- Salima Machkour-M’Rabet View author profile